Page 214 - Spirit and Mind. Vol 1
P. 214
Nicolai Levashov. Spirit and mind. Vol.1
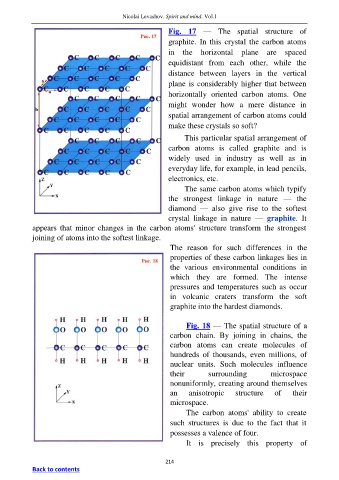
Fig. 17 — The spatial structure of
graphite. In this crystal the carbon atoms
in the horizontal plane are spaced
equidistant from each other, while the
distance between layers in the vertical
plane is considerably higher that between
horizontally oriented carbon atoms. One
might wonder how a mere distance in
spatial arrangement of carbon atoms could
make these crystals so soft?
This particular spatial arrangement of
carbon atoms is called graphite and is
widely used in industry as well as in
everyday life, for example, in lead pencils,
electronics, etc.
The same carbon atoms which typify
the strongest linkage in nature — the
diamond — also give rise to the softest
crystal linkage in nature — graphite. It
appears that minor changes in the carbon atoms' structure transform the strongest
joining of atoms into the softest linkage.
The reason for such differences in the
properties of these carbon linkages lies in
the various environmental conditions in
which they are formed. The intense
pressures and temperatures such as occur
in volcanic craters transform the soft
graphite into the hardest diamonds.
Fig. 18 — The spatial structure of a
carbon chain. By joining in chains, the
carbon atoms can create molecules of
hundreds of thousands, even millions, of
nuclear units. Such molecules influence
their surrounding microspace
nonuniformly, creating around themselves
an anisotropic structure of their
microspace.
The carbon atoms' ability to create
such structures is due to the fact that it
possesses a valence of four.
It is precisely this property of
214
Back to contents